This article first published in the Financial Times in September 2016.
The news that Facebook founder Mark Zuckerberg and his wife Priscilla are putting $3bn towards “ending all disease” has renewed the spotlight on the giving of Silicon Valley’s wealthiest. They are not alone in their enthusiasm for medical research: it is also the most popular target of charitable giving in the UK. 
There is a problem, however: about 85 per cent of medical research is wasted. That’s equivalent to 22 of the 26 miles which people run in marathons to raise funds for research into cancer and other conditions. Globally, that waste costs around $170bn every year. Perhaps many of us suffer with conditions which could have been cured if those funds had been spent more productively.
The startling statistic comes from the academic work of health experts Sir Iain Chalmers and Paul Glasziou in Oxford and Eastern Australia respectively. They identify four main causes of waste and suggest a number of ways in which we might fix them.
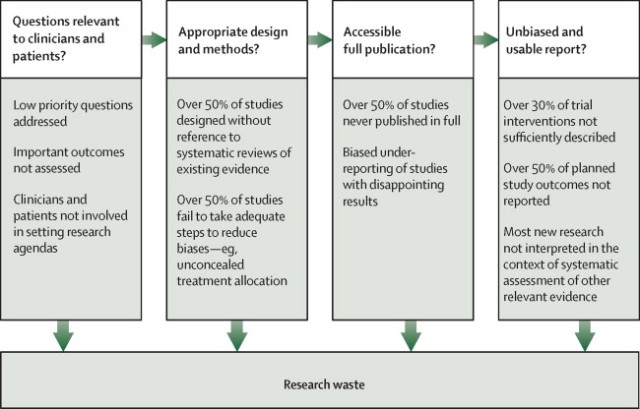
First, wrong question. Much research addresses questions to which the answer may already be known: fewer than half of medical trials appear to be informed by all the previous relevant research. Another slice of research asks questions which are of little or no interest to patients. For example, only 9 per cent of people with osteoarthritis in the knee want more research into drugs, though over 80 per cent of trials for that complaint are drug-focused. Such mismatches occur for many types of medical problem. And right across the board, the most researched problems are often not the most severe or most prevalent.
Second, unreliable research methods. Many trials fail to compare an intervention with anything — and in my last column we saw how misleading that can be — or are conducted on too few patients. The average trial tests an intervention on only 36 people — clearly way too small to say anything statistically robust. (Incidentally, Emily Sena of Edinburgh University suggests that people interested in reducing the number of animals in pre-clinical research should, ironically, push for trials with more animals. Many studies have too few animals to be useful — taking the animals’ lives to no end — whereas fewer studies with more animals might enable researchers to discover something useful.)
Third, research is not clearly reported. For example, clinical research does not always adequately explain what the intervention being examined actually was. Does the patient see the same psychotherapist in every session, or do they change around? How often are the sessions, and how long are they? What is the dosage of the medicine, and how often do people take it? Was the surgery carried out on frail old ladies or strong young men, because you’d expect their survival rates to be quite different? If the research purports to provide a “recipe” for creating the alleged results, any doctor reading it needs that recipe in some detail.
And fourth, often research isn’t reported. Anywhere, ever. About half of all clinical trials are literally never published. Health systems and taxpayers pay dearly for this. In 2009, for example, governments around the world were concerned about a flu epidemic and stockpiled the drug Tamiflu made by Swiss company Roche. The UK government alone spent £500m on it. A Japanese researcher noticed that of the 10 studies on Tamiflu, eight had not been published. After a long fight, enough data about those unpublished trials were released to show that Tamiflu may not reduce deaths and does not even reduce hospitalisations.
Worse, it is not just a random half which never see the light of day. Studies funded by pharmaceutical companies (which have a strong incentive to show that their products work) are four times less likely to show a negative result than are independent studies. Studies with negative findings tend to take a year longer to publish than those with positive findings. Studies funded by charities and private donors fare only marginally better:
 [Graph from The Lancet series on research waste, ‘Increasing value, reducing waste 4‘]
[Graph from The Lancet series on research waste, ‘Increasing value, reducing waste 4‘]
Fixing all this is difficult. Health research is a “system”, where behaviour is driven by incentives, infrastructure and information. The incentives for academics and pharmaceutical companies do not always align with those of patients and the public.
But if you support medical research through charitable donations and foundations, you have some powerful levers. You can ensure that the medical research organisation is aware of these problems (they are well-documented in a series in The Lancet) and has systems in place to address them.
For example, there should be a process for ensuring that research addresses patients’ priorities. The James Lind Alliance, which is affiliated to the NHS, holds lists of patients’ priorities in asthma, Parkinson’s, depression, end-of-life care and other topics.
You can require researchers to publicly explain how they decided on their research question, their method and the sample size, and to “pre-register” (that is, specify publicly) their research plan and publication timetable — this effectively commits them to publishing it.
And you can stipulate that research which you fund or participate in stands on the shoulders of everything that already exists and is under way — and helps others in turn to stand on its shoulders — by detailing the researchers’ actions and results, and ensuring all the resulting data are freely accessible.
These steps may sound blindingly obvious — but they don’t happen by magic and could save many lives.

